Note for contributors: This document is a living research effort. We welcome feedback and discussion!
Translation: From Disease Treatment to Early Intervention
Rotation Opportunity
Develop early interventions prior to disease development to prevent onset and help each person reach their full health potential.
The Legacy
Key Insight: Biotech has mastered late-stage disease intervention but lacks incentives for pre-disease targets, leaving a $5T+ wellness market to fill the gap.
Through sheer grit and determination, the biomedical industry has proven that new molecular targets of the disease state can open entirely new market opportunities. In the field of oncology, an immunological approach was viewed with skepticism after failed trials, but was reborn with the discovery of immune checkpoints and validated by ipilimumab's approval in 2011 [1]. Similarly, gene therapy faced decades of doubts until CRISPR-based therapies like exa-cel showed curative potential for sickle cell disease and β-thalassemia in the early 2020s [2]. Obesity, once dismissed as a lifestyle issue, was reframed as a treatable chronic disease after semaglutide demonstrated 15% sustained weight loss with a favorable safety profile [3]. Such breakthroughs brought about skyrocketing markets for immuno-oncology, gene therapy, and peptide therapy for treatment of diseases, but not without a decade or more of heavy scrutiny.
Biotech has had insufficient incentives to identify and develop adequate therapeutics for targets in the pre-disease state. Blood cholesterol is a prime example – first discovered as early as the 1950s, high low density lipoproteins (LDL) cholesterol was established as a risk factor of cardiovascular disease. However, cholesterol testing did not become standardized and routinely implemented until statins were popularized to lower "bad" LDL levels in the 1990s and early 2000s. In more recent years, there have been criticisms of the literature and indications that statins may have low efficacy in preventing cardiovascular disease, despite reducing LDLs. Still, there are other protective effects of statins that are less-well understood, such as reducing arteriosclerosis, diabetes, inflammation and even cancer. The growing market for statins is indicative that there is a demand for proactive medicines to counteract high risk of disease.
With so few biomarkers and interventions to prevent disease, patient consumers have expressed their frustration, seeking alternatives for more proactive medicine. The wellness industry has surged in response, now projected to exceed $5–6 trillion globally, encompassing sectors such as wearable health monitoring, personalized nutrition and microbiome testing, hormone and metabolic optimization, air and water purification technologies, at-home biomarker testing, and exposure-avoidance products marketed for "detoxification" or longevity. While the evidence base for many of these interventions remains limited, these are strong market signals that consumers are willing to pay out of pocket to detect risk earlier and mitigate chronic stressors. The biotech ecosystem will inevitably respond to market demands, and could foreseeably include environmental exposures.
Major funding sources for human studies have traditionally endorsed research on single exposure-disease relationships, with few if any dollars going to multi-exposures or multi-disease outcomes. Not only is this antithetical to general knowledge from years of biomonitoring studies of exposure, but it also undervalues the disease risk found in typical epidemiological studies. In the largest and most renown human epidemiological studies, the attributable risk of a single disease due to a singular given exposure rarely accounts for more than a doubling of the underlying probability. Therefore, an odds ratio of 2.0 indicates that there is twice the disease risk in the exposed compared to the unexposed. If the underlying disease risk is only 1% in the population, the attributable risk in the exposed group is an extra 1%. This relatively low measure of effect makes it difficult to justify further action—whether it be regulatory, public outcry, or even further research on disease etiology. As a result, the field comes to a standstill.
Figure: Forest plot of epidemiological studies showing environmental exposure-disease associations. Effect estimates (hazard ratios, odds ratios, relative risks) are displayed on a log scale, with the vertical line indicating no effect (1.0). Filter by disease category, chemical class, or study type to explore patterns.
Overriding the one-for-one exposure:disease identity would lead to more accurate depictions of disease etiology and would reconfigure the arcane policy and research funding structures that have held back the field. A single exposure could lead to numerous outcomes and multiple exposures can contribute to the same outcome. For diseases, cancer is shown to be linked to exposure to heavy metals, pesticides, and air pollution while reproductive outcomes are associated with endocrine disruptors. PFAS is linked to thyroid disease and immune dysfunction, while pesticide exposures are associated with neurodegenerative disease and metabolic disorders. The 1:1 exposure-disease relationship downplays the interconnectedness of the exposure risk across body systems and over time.
Studies on resilience against environmental exposures and identifying biomarkers of early effect have been explored in academic contexts. Chemoprevention against pollutants has surfaced in the academic literature for over two decades. Studies on the relationship between specific proteins in protection against adverse effects of toxins have shown some promising results in vitro and in vivo, but failed to translate these results to high impact findings in randomized control trials. For example, Nrf2 or PON1 are responsible for anti-inflammatory and antioxidant responses to toxicants with downstream implications. Despite the potential for wide-acting effects, these fragmented findings never gained traction and are deprioritized in favor of molecular targets that are disease-specific and for late-stage treatment.
Rotating into a New Position
Key Insight: GLP-1 agonists prove that multi-system targets with diffuse receptors can yield blockbuster results—a model for environmental health interventions.
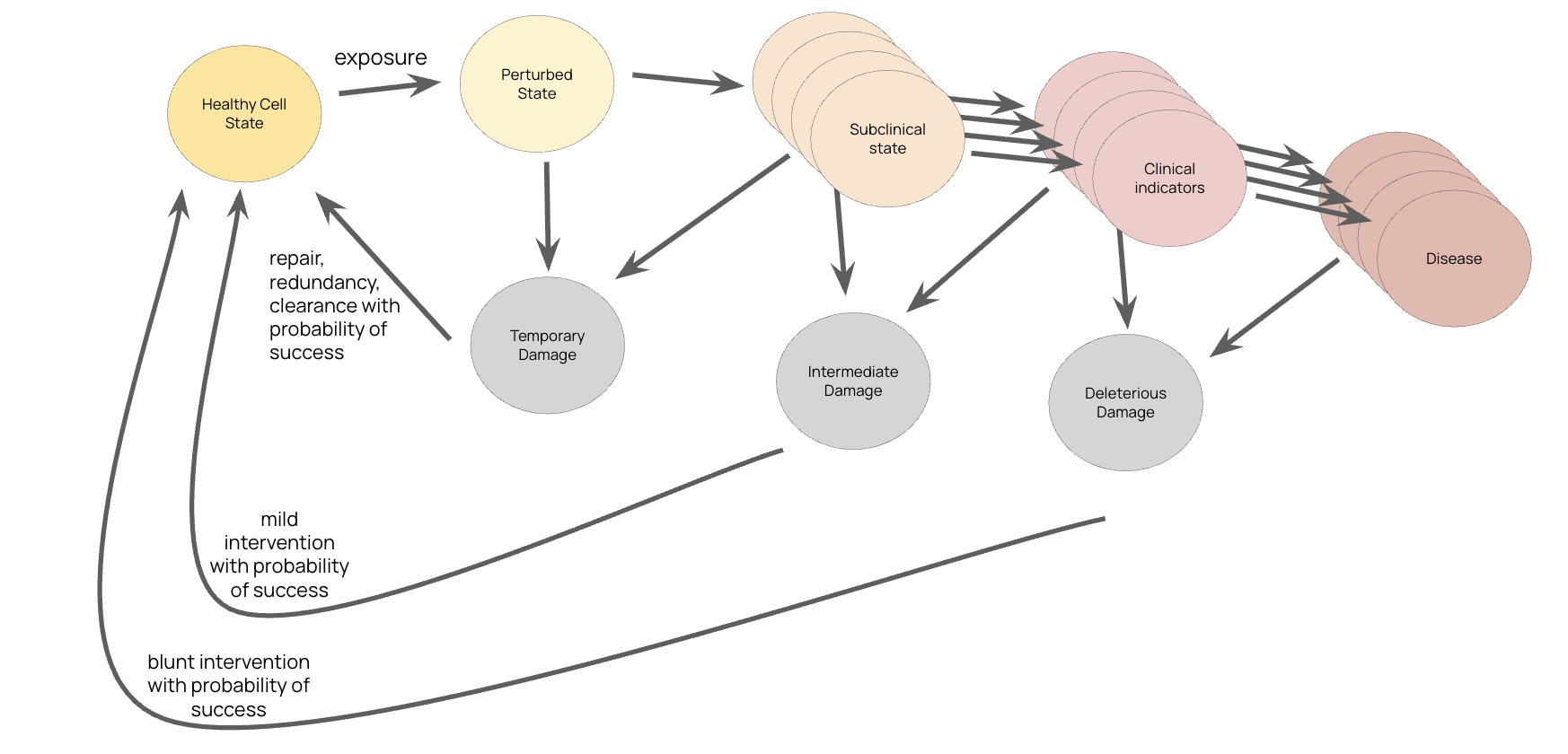
Figure: The progression from healthy cell state through perturbed, subclinical, and clinical states to disease. Cells have natural repair, redundancy, and clearance mechanisms. Early "mild interventions" have higher probability of success than late-stage "blunt interventions" after deleterious damage has occurred.
The pharmaceutical world has opened a new window of opportunity in successfully intervening on interrelated systems of the body at earlier stages of pre-disease. The newest phenomenon of drug class, GLP-1s, have a more fine-tuned modulating effect across multiple systems beyond just metabolic. The GLP-1 receptor was discovered in the 1980s through the study by an endocrinologist seeking to understand the effects on the pancreas by a little known toxin from the gila monster lizard. After decades of research, GLP-1 receptors were shown to be somewhat redundant (i.e., there is no phenotype for a knockout mouse), they nudge the insulin pathway with moderation and few side effects.
The surprising effects of GLP-1s beyond glucose regulation and adiposity, including evidence of cardiovascular, kidney, and neuro effects, are due in part to the fact that the receptors are diffuse and expressed across many tissues including the heart, kidney, immune cells, and the brain. GLP-1 agonists revealed that these conditions share upstream regulatory biology accessible through a single molecular target distributed across gut, pancreas, brain, heart, and immune system. With the anticipated anti-inflammatory effects on a variety of tissues, the valuation of semaglutide is set to be a record-breaking win of this generation for biotech.
The models developed for studying the multi-system-level circuitry for endocrine-signaling drug classes (e.g., peptides) could be transferred to the field of environmental health. Finding the common denominators of molecular perturbations from classes of pollutant exposures may shed light on the main wiring for a multitude of different disease states.
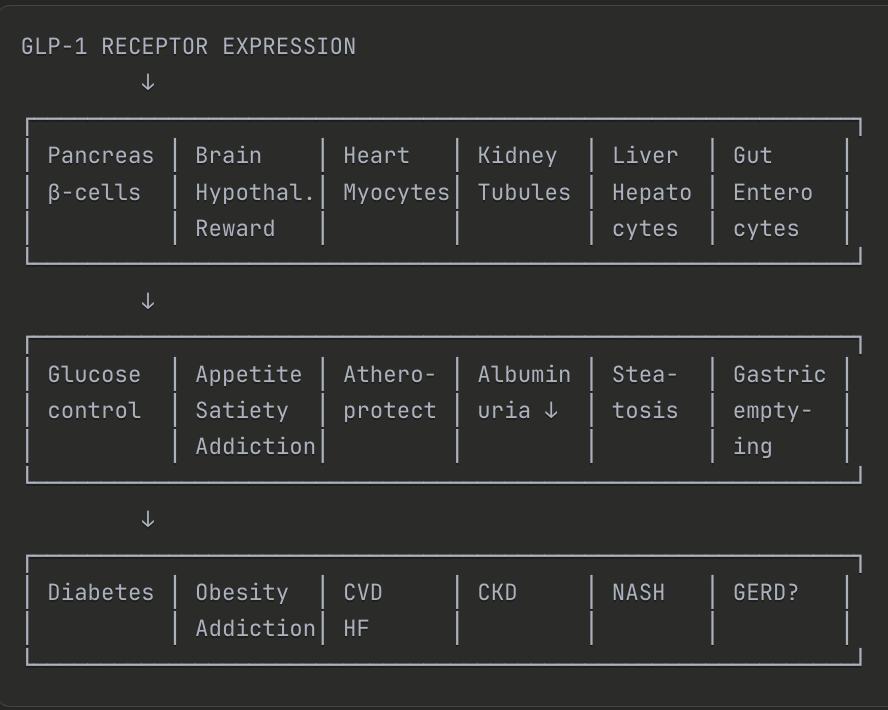
Figure: Visual comparison of GLP-1 receptor hitting multiple targets across organ systems vs. anti-toxin interventions hitting multiple targets for multiple disease outcomes.
Studies on toxins related to neurodegenerative disease (ND) may provide some clues. Pesticide exposure results in hampered mitochondrial activity, all which have shown to be related to a variety of neurological outcomes. Still, multiple NDs are rarely studied in unison with pesticide exposure, yet new relationships between these exposures and diagnosis that increase risk of NDs are frequently reported.
There is a recent trend in NDs being recharacterized based on biomarkers and prodromal effects as opposed to the clinical disease outcomes, and so better profiling of environmental factors with early mechanisms of action could have added value creation for early detection and new treatment regimens. Beyond NDs, pesticide exposure is also associated with other outcomes including diabetes, certain cancers, and reproductive disorders. The interdependency of protein networks may allow these interventions to have a positive spill over effect to reduce risk of other disease states, as demonstrated by the prospects of semaglutide.
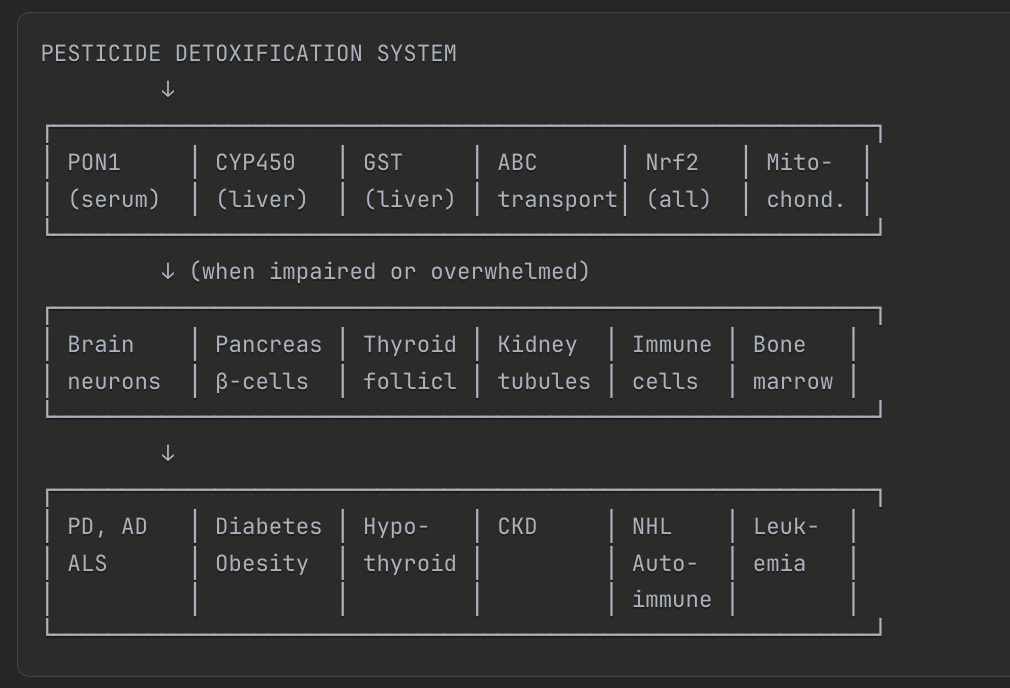
Figure: The pesticide detoxification system involves multiple enzymes (PON1, CYP450, GST, ABC transporters, Nrf2, mitochondria) that, when impaired or overwhelmed, can lead to damage in multiple organ systems—contributing to conditions including Parkinson's disease, Alzheimer's disease, ALS, diabetes, obesity, hypothyroidism, chronic kidney disease, autoimmune disorders, and leukemia.
More broadly, reconfiguring the definitions of chronic illnesses and conditions based on earlier states and environmental exposures could contribute to a new iteration of risk scores. The gene-by-environment (GxE) interaction framework was initially proposed, but there was little statistical power to support that this was a strong finding, as much of the work has been examining single exposures at a time. With study sizes and cost/compute efficiency increasing by orders of magnitude in recent years, the relationship between inheritable and noninheritable factors of disease have been further elucidated. The remaining unexplained variability in existing models could be improved by including environmental exposure data.
Second Order Effects
Key Insight: Environmental exposure research illuminates new pathways (like AhR) that become drug targets, and enables disease subtyping for precision medicine.
New mechanisms that emerge from the study of environmental exposures can have positive externalities on the clinical field. For example, the discovery of AhR, made possible by the study of dioxin exposure, has led to its emergence as a drug target in psoriasis and immunology [4]. Similarly, chelation therapy, which was originally developed for acute lead poisoning, has shown secondary benefits of improving cardiovascular outcomes and may even decrease cancer risk through modulation of pathways like p53 [5]. By furthering the study of environmental pollutants, we can expand this second order effect of illuminating biological pathways that lead to new prospective treatments.
Including environmental exposures as an integral part of disease etiology would serve as a starting point to redefine chronic illness based on molecular changes that are upstream of the disease onset. This would have a profound impact on new approaches to high-resolution profiling of disease states, to profile and subtype for more specialized treatment. For example:
- Asthma: The Diagnostics Accelerator at Harvard's Wyss Institute has subtyped an asthma patient population based on proteomic profiles from mass spectrometry data
- Endometriosis: A detailed subtyping effort based on electronic health records, surgical metadata and omics analysis could lead to better categorization of the chronic condition
- Parkinson's disease: Prodromal subtypes are being identified that could enable earlier, targeted intervention
With known environmental factors that contribute to asthma, endometriosis, and Parkinson's disease, there is added benefit to enhancing these datasets with measurements of past or current environmental exposure to move towards a more comprehensive and fine-tuned segmentation procedure of the diseased state, and work backward to ultimately identify and intervene at the pre-disease state.
Benchmarks to Hit
Roadmap: A seven-year path from cohort establishment to FDA-approved prophylactic therapies.
- Year 1: Establish cohorts for longitudinal biomarker tracking of exposure-response relationships
- Year 3: Validate at least three surrogate endpoints for exposure-related pre-disease states
- Year 5: Achieve FDA acceptance of at least one prevention-oriented molecular target for environmental exposure
- Year 7: Launch first approved prophylactic therapy targeting environmental exposure pathways
References
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-23.
- Frangoul H, Altshuler D, Cappellini MD, et al. CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. N Engl J Med. 2021;384(3):252-60.
- Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002.
- Silverberg JI, Boguniewicz M, Hanifin J, et al. Tapinarof cream 1% once daily for the treatment of moderate to severe atopic dermatitis in adults and children. J Am Acad Dermatol. 2024;90(1):65-73.
- Lamas GA, Goertz C, Boineau R, et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 2013;309(12):1241-50.